Following my industrial internship practicum in plant biotechnology, I became fascinated with regnerative biology. I wanted to explore this area further, hoping to eventually work on a model for organogenesis in order to develop "organ-in-a-dish" where transplantation-ready organs can be made to order. In October of 2005, I joined the laboratory of Prof Hasan Uludag in the Department of Chemical and Material Engineering at the Universty of Alberta. Prof Uludag specializes in synthetic biocompatible polymer for the delivery of growth factors as a potential treatment for osteoporosis.

My first project was to investigate whether bone regeneration can be mediated by gene delivery of a DNA vector coding for the osteoclastogenesis inhibitory factor (Osteoprotegerin) in bone marrow stromal cell.
After a relatively productive smooth-going first 6-months in the lab, I hit a major technical roadblock - I was not able to get any appreciable bone mineralization from cells grown in tissue culture. It wasn't until many failed attempts that I realize that the delvery of DNA to tissue derived cells is a major rate-limting step. The challenge then was to figure out whether it's because the DNA was not getting into the cell or if the cells were not able to utilize the DNA efficiently once its in. Thus began a painfully long 3 years of troubleshooting, experimenting, and optimizing thousands of variables trying to work out where the most signficant barriers were.
It seemed like an impossible task at the time because I had no idea whether the experimental system we were working with had any hope of working at all. The initial results were quite demoralizing, maybe a 2-3% increase in efficiency. I thought about quiting graduate studies and research altogether many times. But bit by bit, small margin of increase in efficency here and there eventual build up to something more significant. After thousands of experiments, testing hundreds of variables in different configurations, I eventually figured out that the way these DNA-polymer complexes were formed played a huge part in how well the cells were able to internalize and express the gene of interest.

With a work protocol in place, I began to systematically decipher the intracellular processes that govern how these DNA complexes are be processed by the cell. Using a combination of inhibitors that block different parts of the intracellular pathway, I worked out several distinct intracellular processes that these DNA complexes go through, from binding to the cell surface, to translocatng across the nucleus, to the evenual expresson of the protein that we designed. I summarzed my findings a paper that is now published in the journal"Biomaterials."
Through the countless experiments and problems encountered throughout my research, I gained an unprecedent insights into the intracellular processes and pathway involved in the uptake and expresson of exogenously introduced DNA. I summarized everythiing I have learned in my PhD into one revew artcle and further proposed how a modular gene delvery system can be designed to circumnavigate the trick intracellular environment. This review paper is published in the Journal of Drug Targeting.
Following the completion of my PhD, one of my supervisory committee member was looking for a postdoctoral fellow for a collaboratve research team to work on the bioprocess development of stem cell-derived cardiomyocytes. I joined the team as a senior trainee/project manager and applied my gene delivery expertise to reprogram somatic cells into pluripotent stem cells in the bioreactor.
As my postdoctoral fellowship was designed to be a transition step towards become an independent research scientist, I was tasked wth the floor operation of the lab as well as supervsion of graduate students, in additon to applying for grants and develop my own research program

The research program that I was working on was largely inspired by the recent FDA approval of two CAR T-cell based therapies for the treatment of blood cancer. In this novel therapy, patient's own cells are extracted from the blood, genetically engineered with therapeutc propertes, then infused back to the patient to actively reverse the disease process. Cell therapy, unlike traditional biopharmaceutical agents, are living tissues that can seek out, respond, and exert action directly on the affected tissues. It's a completely new modality of treatment. However, these first-generation cell therapy products come with a hefty price tag of about $688,000AUD.
The reason that cell therapy products are so expensive is because 1) the genetic engineering step is a very specialized and laborious process that is currently done manually by a trained technologist. It's akin to making a personalized high performance sports car for one person, which cost a lot to set up. The customized product also can't be used for other people since it's specifically matched to that one individual, so the cost can't be distrubte across a larger pool of user either. 2) The second reason is because there's no well established bioprocess to expand cells into clincally relevant quantities. The current process in place is adapted from the manufacturing of antibodies, which is quite different than cell therapy products.
My postdoctoral research is focused on integrating these two discontinuous processes into one seamless, automated bioprocess so that the overall biomanufaturing of cell therapy products can be streamlined and scaled out. The first step of the research was to see if the cells can be genetically modified when grown in a stirred suspenson bioreactor. And the answer is yes, cells can be transfected even when they are spinning in a batch of liquid media in a stir tank. Efficiency is even better in the bioreactor because their growth rate is much faster than in a dish. This finding is published in Molecular Therapy - Methods and Clinical Development
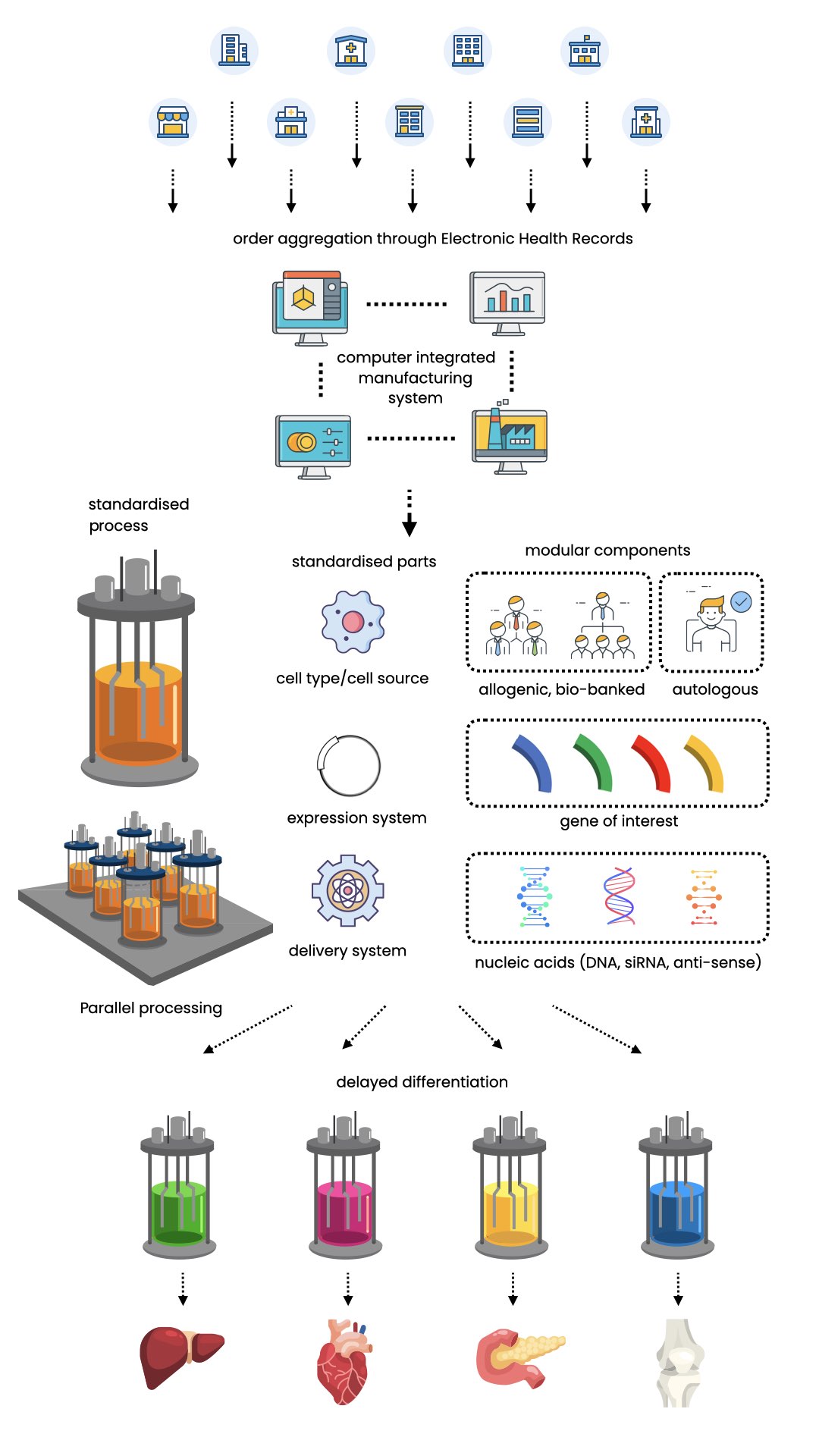
Towards the end of my tenure as a postdoc, I was working on commercializing my method into a platform technology. Because gene delivery is a tool to introduce DNA into the cell, the process can be modularized - meaning an interchangeable set of vectors, expression cassettes or nucleic acid molecules can be swap in and out to impart different properties in a cell. This then allow a product family to be developed around a set of core technology - a platform.

I had envisioned this platform technology to be implemented as a mass customization platform, where a large combination of patient-specific cell therapy products can be derived from a finite set of process with interchangeable components. I entered a number of start-up competitions (picture above, my booth at the TENET I2C business pitch competition) to raise some seed funds. I had planned to incorporate a company to start securing government grants and approach venture capitals. I was offered a space at a new incubator/accelerator space in South Calgary early 2016 to start my own lab. But at that point I was exhausted. I was working on the project by myself, carrying the entire weight of the concept on my shoulder. I also thought the research environment and the tech culture in the area was not right for this type of work. So I made the decision to take a break from it and study medicine in Australia instead. Maybe I will come back to it, maybe I won't. But I hope some day, in the near future, I will be able to prescribe cell therapy products in my medical practice.
Below is a schematics that I have put together, of the mass customization platform that I had envisioned